Post 3: Physiological interpretation of the diaphragm electrical activity (EAdi) waveform.
Breathing is an extremely complex process and we are still learning. The EAdi waveform adds a new dimension to interpreting breathing at the bedside. It provides information about central respiratory drive, timing of breathing, and synchrony between ventilator assist and patient effort. This blog will describe areas where EAdi provides important physiological information. Importantly, EAdi waveform is equally informative and reliable regardless if monitored during invasive or non-invasive ventilation.
EAdi to evaluate central respiratory drive
Basically, breathing is a vital function that enables us to acquire O2 and eliminate CO2, the later of fundamental importance for regulation of pH. It is an involuntary process usually referred to as the central respiratory drive, responding to changes in chemical milieu, load, stress, emotions, speech etc. Also, central respiratory drive can also temporarily be voluntarily overridden. Before the availability of EAdi at the bedside, it was difficult to interpret the central respiratory drive and factors that affect it at the bedside. Even more so when a patient is mechanically ventilated.
The EAdi, is a cumulated output signal with its origin in the central respiratory centers. It is transmitted as packages of electrical impulses propagating along the phrenic nerve fibers that ends up innervating groups of muscle fibers forming so called motor units at the level of the diaphragm. Transmitted via the neuromuscular junctions, single fiber action potentials are initiated to propagate along diaphragm fibers. As the phrenic nerves innervates the diaphragm in motor units packages, the EAdi represents motor unit electrical activity, and its energy can be measured in the time domain as a temporal and spatial summation of electrical activity (see previous postings for more details). Moreover, the propagation velocity and frequency domain components of the action potentials can also be measured and used for more detailed information about muscle properties and fatigue.
Unless the neuro-muscular transmission of electrical signals is not faulty, the EAdi will reflect central respiratory drive within a given subject in the following manner:
- No EAdi signal suggests central apnea i.e. loss of central respiratory drive.
- Presence of EAdi suggest presence of respiratory drive, and within an given subject, the higher the EAdi amplitude the higher the central inspiratory drive.
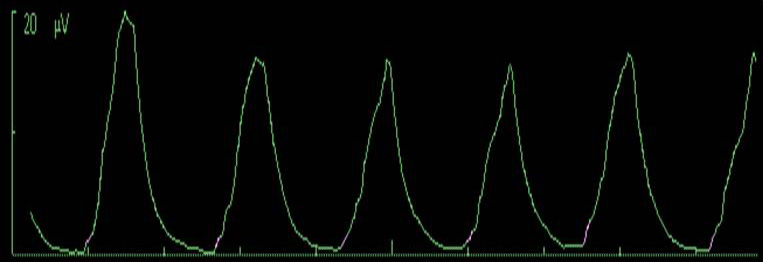
With breathing, the EAdi is typically cyclic representing a “phasic” activation of the diaphragm. The higher the frequency of the phasic EAdi (i.e. higher neural respiratory rate) the higher the central respiratory drive. The EAdi can also be continuous representing a “tonic” activation of the diaphragm. The tonic and phasic EAdi can add to each other. Hence, it is also relevant to evaluate central respiratory drive as a time integral of Edi.
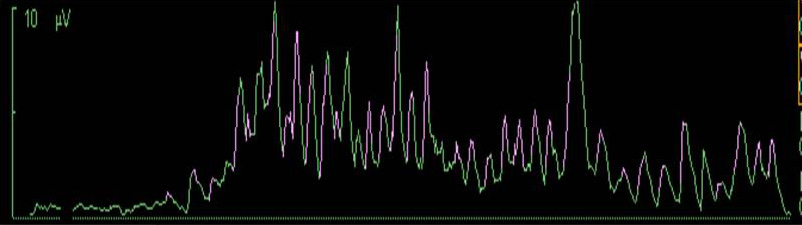
Figures below shows phasic EAdi waveforms in adult and no EAdi followed by phasic on tonic EAdi in newborn.
No EAdi
Given that EAdi is a summation signal it does have a true zero (note that this requires proper electrode positioning, signal acquisition, processing and noise control). Absence of the EAdi therefore indicates loss of central respiratory drive. Hence, EAdi monitoring allows proper monitoring of central apneas a feature of special importance in preterm and newborn babies where central apneas are frequent.
Note that the EAdi can also be of use in patients with obstructive apneas (presence of EAdi but no inspiratory flow).
Given that mechanical ventilation and sedation reduce the central respiratory drive, monitoring of the EAdi can be helpful to ensure appropriate clinical interventions. Likewise, monitoring to ensure absence of EAdi during use of paralytic agents can also be of value.
Monitoring EAdi can also be useful post-operatively to determine when spontaneous breathing is restored.
Phasic EAdi
Increased respiratory demand is typically generated by increased metabolism and increased need to eliminate CO2 and will, within a given subject, cause the EAdi signal to increase in amplitude and also increase in frequency (neural respiratory rate). In particular, monitoring the change in the EAdi amplitude in response to devices that increase deadspace can be helpful to avoid unnecessary ventilatory demand.
Increasing inspiratory load will increase the amplitude of the EAdi, however this may not necessarily be associated with an increase in the neural respiratory rate. For example the monitoring changes in EAdi amplitude can be of value for the bedside evaluation of load and dead space of heat-moisture exchangers and humidifiers.
Weakness of respiratory muscles (of non-degenerative nature) would also require more motor unit recruitment and/or firing rate, increasing the EAdi amplitude.
Improvement of ventilation and unloading of respiratory muscles is the prime target for both applying PEEP and mechanical ventilation. Successful application of PEEP to overcome intrinsic PEEP, should reduce the EAdi amplitude. Likewise should application of mechanical ventilation reduce the EAdi amplitude if it achieves its targets to improve ventilation and reduce work of breathing. Note also that during mechanical ventilation the EAdi waveform also allows bedside evaluation of patient-ventilator interaction. This opens an interesting opportunity to determine the origin of the reduction in EAdi amplitude. On the one hand, ventilatory assist that is synchronized to patient effort acts to improve ventilation by sharing the load with respiratory muscles allowing the patient to determine the degree of ventilation. On the other hand, mechanical ventilation that is not synchronized to patient effort still acts to increase ventilation, removing CO2, and decreases the respiratory drive without considering the patient’s own respiratory regulation. Monitoring patient-ventilator interaction and change in EAdi opens the possibility to identify these two extremes of decreasing central respiratory drive.
The influence of sedatives on respiratory drive can be significant and monitoring the change in the EAdi amplitude at the bedside will illustrate the patient’s response to sedatives on central respiratory drive.
In patients with neuromuscular disorders the EAdi can be used to monitor the progress of the impairment. Impairment of central drive, impaired nerve transmission, neuromuscular transmission, or muscle excitation, results in a diminishing EAdi signal. However, it should be noted that reinnervation (e.g. after poliomyelitis) may enlarge the EAdi signal due to enlarged motor units.
Tonic EAdi
In certain patients, the EAdi persists between phasic periods i.e. the diaphragm is active also throughout the exhalation period. This is referred to as tonic EAdi, and has been observed during removal of PEEP and application of negative pressure to the airways and is therefore assumed to be induced by lowered endexpiratory volume and lung derecruitment.
See also these conference videos: